Latest Articles
The latest news and information regarding electronic medical records, practice management software, HIPAA, and security from Nextech.
Courtney Tesvich serves as Nextech's VP of Regulatory and Compliance.

Ophthalmology | Compliance | CMS | Coding | Clinical Efficiency
By:
Courtney Tesvich
November 21st, 2025
The odds of a healthcare practice being audited took a tremendous jump this year. Proactive administrators are taking action now to make sure their practice is ready should auditors come knocking.

By:
Courtney Tesvich
June 3rd, 2025
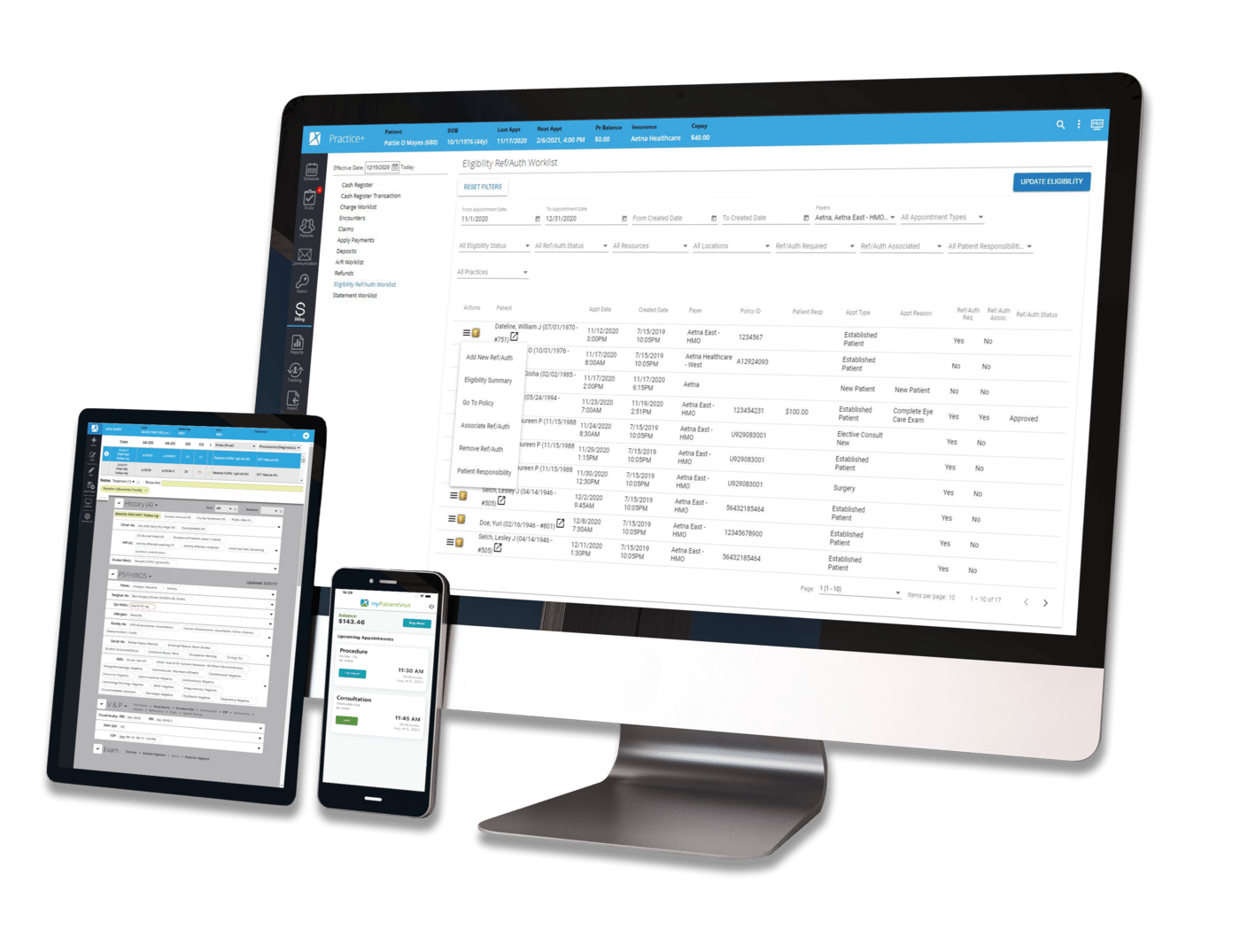
You’re working hard to build a successful practice where patients feel safe, cared for, and respected. Part of earning that trust is making sure their private health information stays private.

By:
Courtney Tesvich
May 29th, 2025
Patient care is at its best when it involves seamless coordination among multiple providers with different expertise. However, there can be challenges with sending and receiving patient information with another practice, especially if their technology isn’t equipped to facilitate smooth collaboration. To keep provider communication flowing, encourage data sharing from one practice to the next, and keep patients more informed about their care, the federal government created regulations around information blocking and other EHR mandates.

By:
Courtney Tesvich
March 12th, 2024
EHR mandates became law in 2016, with enforcement penalties starting in 2024. This may feel like a new requirement, but it’s been 15 years in the making.

Compliance | Regulatory & Compliance | cures act
By:
Courtney Tesvich
November 3rd, 2022
The end-of-year deadline for EHR development companies to comply with the Office of the National Coordinator for Health Information Technology (ONC) Cures Act certification updates is rapidly approaching and you may be wondering what this means for your EHR vendor and for your practice.

By:
Courtney Tesvich
December 7th, 2021
Back in November, the Centers for Medicare & Medicaid Services (CMS) released the 2022 Final Physician Fee Schedule that outlines Quality Payment Program (QPP)/Merit-based Incentive Payment System (MIPS) changes for next year. This blog will provide you with a high-level summary of changes to help you understand how to plan your quality improvement process for next year.

Regulatory & Compliance | cures act
By:
Courtney Tesvich
November 11th, 2021
While a great deal of information has been released on how electronic health records (EHR) will be affected by the Cures Act regulations, there seems to be far less understanding about how the new requirements will affect practices. In this blog article, we will clarify how some of the new requirements will apply to your practice, even if you are not a MIPS participant, as well as how transitioning to the cloud from a server-based system will make complying with these regulations easier for your practice next year and into the future.

By:
Courtney Tesvich
August 18th, 2021
Welcome to our second and final installment of this blog series on important changes in the 2022 Physician Fee Schedule (MIPS Proposed Rule). In Part 1, we took a look at key changes to the Quality Payment Program (QPP). In this blog, we will summarize the new MIPS Value Pathways (MVPs) as well as important changes to the Appropriate Use Criteria and EPCS Medicare Part D Compliance Requirements.