Latest Articles
The latest news and information regarding electronic medical records, practice management software, HIPAA, and security from Nextech.

Regulatory & Compliance | Revenue & Finances
By:
Nextech
January 5th, 2026
Medicare enrollment is a foundational component of successful business for specialty healthcare practices in dermatology, ophthalmology, and plastic surgery. Before a single Medicare claim can be paid, providers and organizations must be enrolled through PECOS, the Provider Enrollment, Chain, and Ownership System.

MedSpa | Regulatory & Compliance | Aesthetics
By:
Nextech
April 10th, 2025
In 2024, intravenous therapy (IV) brought in $2.1 billion in revenue in the U.S. Since the projected revenue is even higher over the next 10 years, opening an IV therapy clinic could be a lucrative endeavor. However, it's only possible to open a clinic – or add the treatment to an existing aesthetic business – if you meet certain licensing requirements.
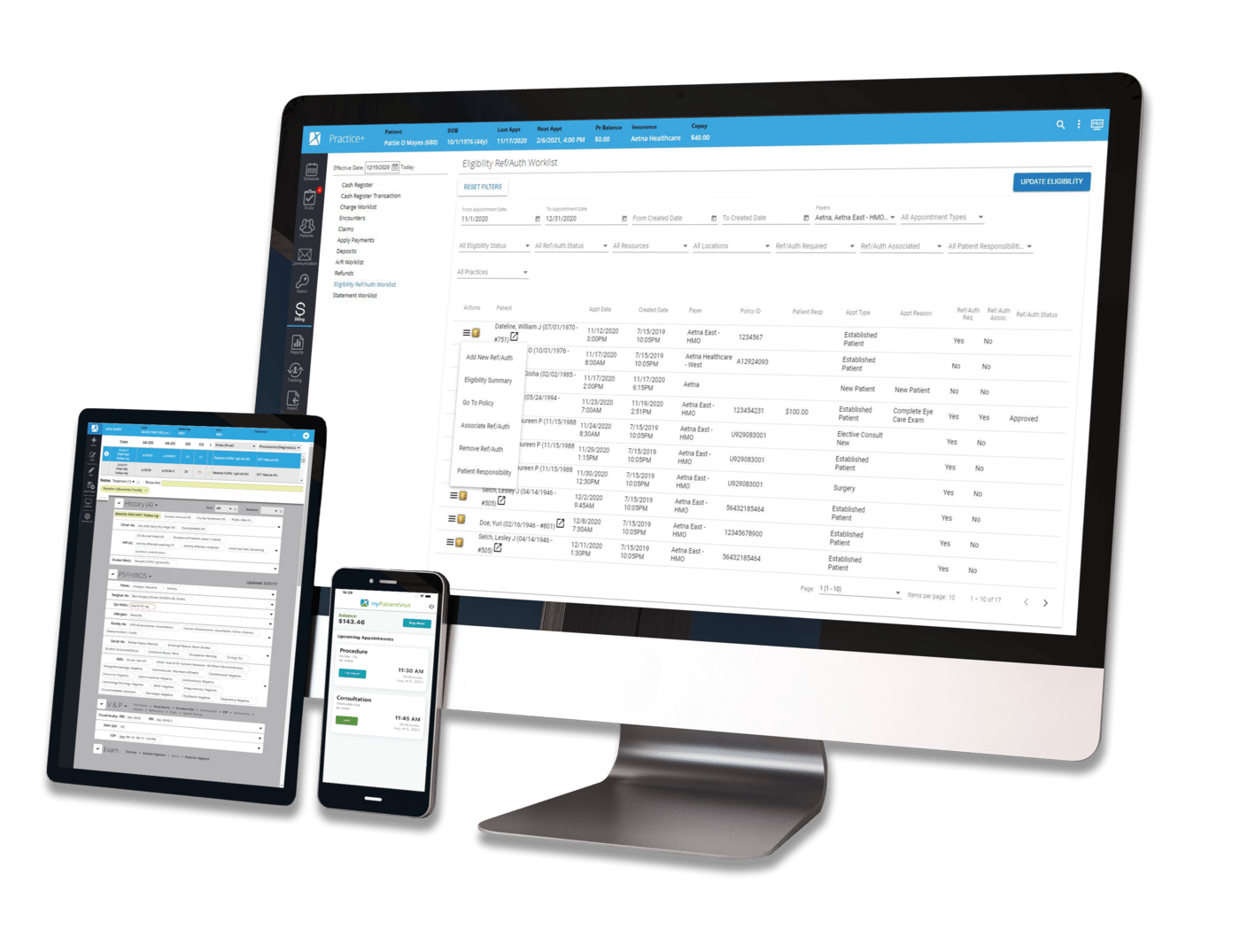

MedSpa | Regulatory & Compliance | Aesthetics | podcast
By:
Tyler Terry
April 24th, 2024
Live from the 2024 Medical Spa Show in Las Vegas, AmSpa founder and CEO Alex Thiersch shares fresh insights on the rapid growth of the industry and an exclusive preview of their upcoming State of the Industry Report. Twelve years ago, Alex’s work as an attorney helping a solo medical spa owner led him to establish AmSpa to offer essential guidance on compliance and safety for others within the industry. Since then, he and his team have introduced much needed resources for med spa owners including boot camps and courses for business, legal, and safety training. With legislative work happening behind the scenes in every state to develop rules and regulations, often without the input of those in the medical aesthetics industry, Alex emphasizes the importance of staying organized and having a seat at the table as new laws are introduced.

Ophthalmology | Plastic Surgery | Compliance | Dermatology | MIPS | Regulatory & Compliance | Orthopedics
By:
Heather Miller Dongilli
November 8th, 2023
Each year, the Centers for Medicare and Medicaid Services (CMS) updates the criteria of the Merit-Based Incentive Payment System (MIPS). In 2026, MIPS is expanding emphasis on MIPS Value Pathways (MVPs) and continuing to refine other measures.

CMS | MIPS | Regulatory & Compliance
By:
Heather Miller
July 24th, 2023
The Centers for Medicare & Medicaid Services recently opened the MIPS 2022 Final Score preview. And already it has led to some confusion.

Ophthalmology | Plastic Surgery | EHR | Dermatology | PM | Regulatory & Compliance | case studies
By:
Nextech
December 12th, 2022
As 2022 comes to wraps, Team Nextech has been busy wrapping up our 2022 case studies. This year, we had 15 case studies spanning three specialties — dermatology, ophthalmology, and plastic surgery. Hear what our clients have to say about Nextech being their chosen solution and why.

EHR | Regulatory & Compliance | Release Party | cures act
By:
Nextech
November 9th, 2022
Before the hustle and bustle of the holiday season settles in, Jack Frost starts to nip at your nose, and you go into your closet for your annual ugly holiday sweater, there’s one thing left to do to stay on the Nice List this year — hang out with Nextech for a digital fireside chat.

Compliance | Regulatory & Compliance | cures act
By:
Courtney Tesvich
November 3rd, 2022
The end-of-year deadline for EHR development companies to comply with the Office of the National Coordinator for Health Information Technology (ONC) Cures Act certification updates is rapidly approaching and you may be wondering what this means for your EHR vendor and for your practice.