

Part 1: Interoperability & EHR Function Measures
On March 20 2015, The Centers for Medicare & Medicaid Services (CMS) released Stage 3 of their Electronic Health Record Incentive Program. At the same time, the Office of the National Coordinator for Health Information Technology (ONC) released the 2015 Editions of the Health Information Technology Certification Criteria, Base Electronic Health Record Definition, and ONC Health IT Certification Program.
This news has resulted in some rather extreme reactions among some in the healthcare industry—for example, absolute panic and unbridled rage. This seems rather odd. Come on… it’s not like we weren’t aware that this was coming. As a matter of fact, I’m pretty sure that I gave everyone a heads up about this back in January.
Based on how certain people are behaving, though, you’d think the CMS & ONC just surprised all of us with this stuff like it’s some kind of weird fire drill. Then again, trying to make sense out of so much complicated (and, let’s face it, very boring) text is probably enough to make anyone a little irritable.
 However, as the title of this article proclaims: Don’t Panic!
However, as the title of this article proclaims: Don’t Panic!
This series on Meaningful Use Stage 3 (MU3) will help you understand the important stuff, without the need to subject your mind to the 300+ pages of rather boring reading it contains. Of course, if you’re the type of person who’d prefer to read the boring stuff… well then, by all means, knock yourself out (the rest of us will see you in a few weeks):
As for those of you who would rather learn what you need to know by reading a couple of short articles (thus maintaining your sanity)… keep calm and come with me.
The Revised Meaningful Use Timeline
The first thing you probably need to know about MU3 is that the CMS has revised and updated their payment and attestation timelines. Below are two very informative tables, the first based on payment years and the other on years of actual participation in the program, taken from the newly released proposed rule:
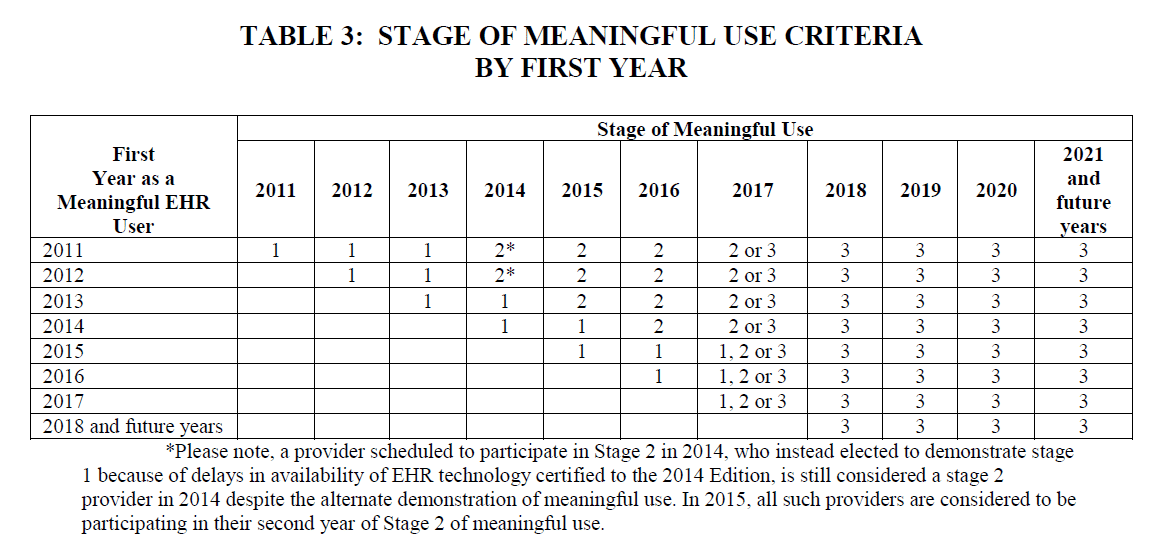
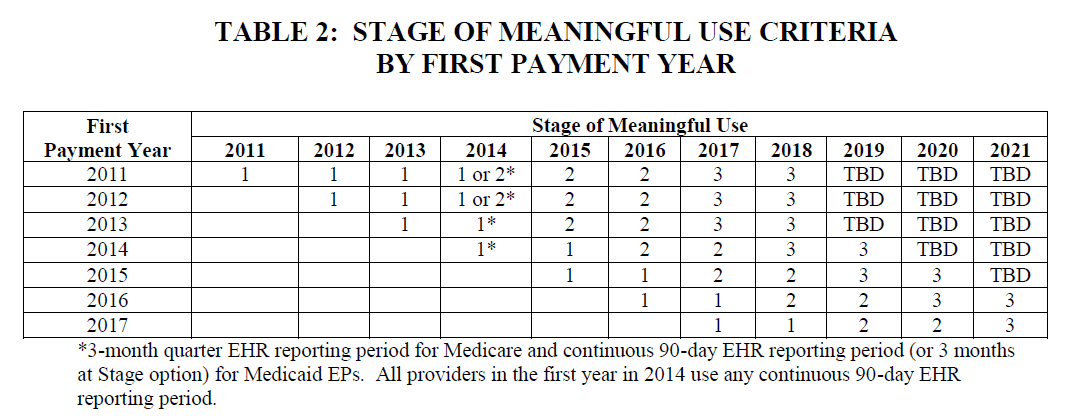
The Primary Meaningful Use Objectives: 2017 and Subsequent Years
The objectives of Meaningful Use, past, present, and future, are broken down into eight primary areas. It’s important to note that each objective is not intended to be singular, and multiple measures are encompassed by most of these. For a (non-hospital) healthcare provider, these eight objectives really contain around fifteen different measures. They also do not include clinical quality measures, which will now be determined via a completely separate process. Unfortunately, the details of that process are not expected to be released until later this year. The objectives/measures of Meaningful Use are explained in the following table, which has been taken from the rule (as released by the CMS):
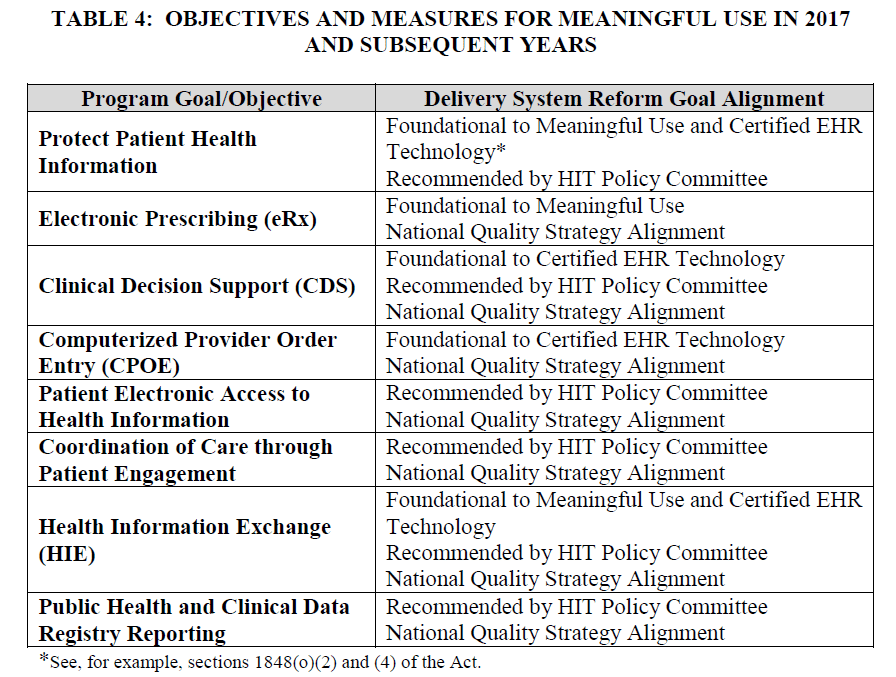
Provider EHR Function Requirements
There are three areas of note when it comes to provider-facing EHR functions—Clinical Decision Support (CDS), Computerized Physician Order Entry (CPOE), and Electronic Prescribing.
Electronic Prescribing
For a non-hospital Eligible Professional (EP), the threshold for ePrescribing has been increased. Now, 80% of all permissible prescriptions written by an EP are to be queried for a drug formulary and transmitted electronically using Certified Electronic Health Record Technology (CEHRT). This measure also includes an exclusion for EPs who write less than 100 permissible prescriptions during a particular EHR reporting period.
Clinical Decision Support (CDS)
This involves two separate measures:
- Requires providers to implement five CDS interventions related to four or more Clinical Quality Measures (CQMs) at a relevant point in patient care for the entire EHR reporting period.
- A consolidation of the “drug-drug/drug-allergy interaction checks” objective from Stages 1 and 2, it requires providers to enable and implement the functionality for drug-drug and drug-allergy interaction checks for the entire EHR reporting period. Also includes an exclusion for any EP who writes fewer than 100 medication orders during the EHR reporting period.
Computerized Physician Order Entry (CPOE)
This involves three separate measures:
- Use CPOE on a minimum of 80% of medication orders
- Use CPOE on a minimum of 60% of lab orders
- Use CPOE on a minimum of 60% of diagnostic imaging orders
Patient EHR Function Requirements
There are two areas of note when it comes to patient-facing EHR functions—Patient Access to Information and Active Patient Engagement.
Patient Access to Information
There are two measures included in this one:
- 80% of patients must be able to access their records via View/Download/Transmit functions, or through some other ONC-certified Application Program Interface (API).
- 35% of patients must be given access to patient education resources. It is important to note that this measures only requires EPs to provide Whether or not the patients actually make use of that access is not a factor in this requirement.
Active Patient Engagement
This area involves three separate measures:
- 25% of patients must actually access their records via View/Download/Transmit functions, or through some other ONC-certified Application Program Interface (API).
- 35% of patients must receive a clinically-relevant correspondence via secure messaging.
- Patient-generated health data or data from a non-clinical setting must be incorporated into certified EHR technology for more than 15% of all unique patients seen by an EP.
Interoperability
There are two areas of note when it comes to interoperability—Health Information Exchange (HIE) and Public Health and Clinical Data Registry Reporting.
Health Information Exchange (HIE)
This area involves three separate measures:
- For 50% of TOCs and referrals, an electronic summary must be sent.
- For 40% of TOCs and referrals, an electronic summary must be received.
- For 80% of TOCs and referrals, med/allergy/problem reconciliation must be performed
Public Health and Clinical Data Registry Reporting
There are six measures included in this one, and EPs are required to choose at least three from measures 1 through 5 (you can ignore 6, which is only for EHs):
- Active engagement is required for immunizations
- Active engagement is required for syndromic surveillance
- Active engagement is required for reportable conditions case reporting
- Active engagement is required for public health registries
- Active engagement is required for non-public health registries
- Active engagement is required for electronic lab reporting
“Active engagement,” as it is used above, has been (rather broadly) defined by the CMS to include registering, testing, or transacting. Therefore, as long as you are at least demonstrating that you are making the necessary effort to comply with these measures, you can still receive credit even if you are not actively transacting.
That just about wraps up this first installment of this series on Meaningful Use Stage 3. If you were panicking, I’m hoping you now feel a bit calmer about the whole thing. If you were already calm, I sincerely hope this information hasn’t caused you to panic (I figure it could go either way). In Part 2, we are going to look at the important highlights of the 2015 Editions of the Health Information Technology Certification Criteria, Base Electronic Health Record Definition, and ONC Health IT Certification Program.
So, remember—don’t panic … we will get through this together.
HERE ARE SOME RELATED ARTICLES YOU MAY FIND INTERESTING
Regulatory & Compliance | Revenue & Finances
What Is PECOS? Step-by-Step Guidance for Specialty Practice Owners
By: Nextech | January 5th, 2026
MedSpa | Regulatory & Compliance | Aesthetics
IV Therapy Laws by State
By: Nextech | April 10th, 2025
MedSpa | Regulatory & Compliance | Aesthetics | podcast
The Current & Future State of the Medical Spa Business with Alex Thiersch
By: Tyler Terry | April 24th, 2024
