Everything You Need to Know About PQRS in 2015
By: Samantha McAlister | April 17th, 2015

Take Advantage of Your EHR
The Physician Quality Reporting System is in full force for 2015, offering several methods of reporting, each with their own rules and standards.
Nextech provides a way for clients to report electronically, allowing a more streamlined all in one report. For 2015, you can align your Clinical Quality Measures for the Meaningful Use EHR Incentive program with the 9 PQRS measures across 3 domains. If you participated in Meaningful Use in 2014, you should already have the measures in your EHR and we’ll be able to pull a report for the 2015 submission process.
Important PQRS Deadlines
If you are reporting as a group for PQRS in 2015, you must register by June 30, 2015. CMS has given step by step instructions on how to register.
If you are reporting individually, there is no registration required.
PQRS is a full-year reporting period and you will submit your measures to the Quality Net Portal in January of 2016. If you report via your EHR, you will generate one report, a QRDA I report for individual reporting or a QRDA III report for group reporting, and submit one time.
Important things to know about 2015 Group Reporting: If you fail to meet the PQRS requirements in 2015, you will be subject to a 2% negative adjustment, along with another 2-4% value modifier negative adjustment based on your practice size and the quality and cost of the measures you reported. These penalties don’t even include the Meaningful Use adjustments for non-compliance, so the penalties can really add up. Let Nextech help you remain compliant and get the most out of your Medicare claims.
Actions Steps:
- Align Meaningful Use and PQRS for 2015 (this is not a formal process – just make a decision as a practice).
- Choose to report via Nextech
- Make sure you register if you are reporting as a group. The following slides offer step by step assistance in registering, as well as everything you need to know about the penalties and possible incentives for PQRS compliance.
- Choose your measures! Nextech recommends the following measures per specialty:

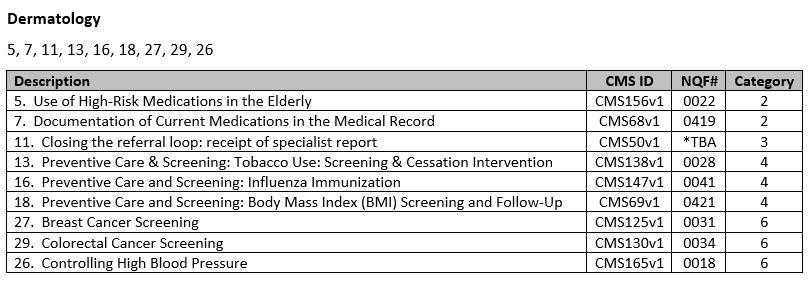
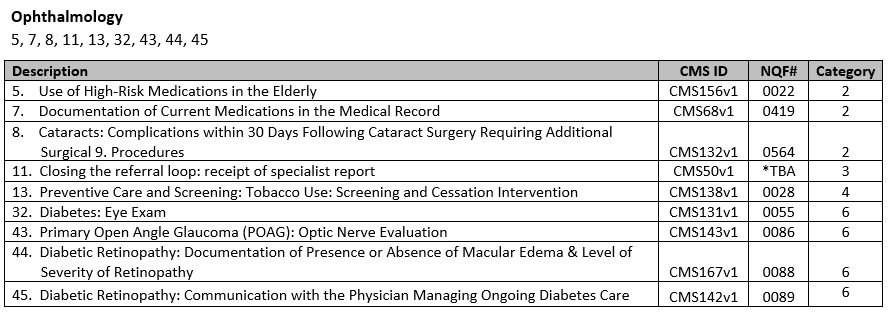
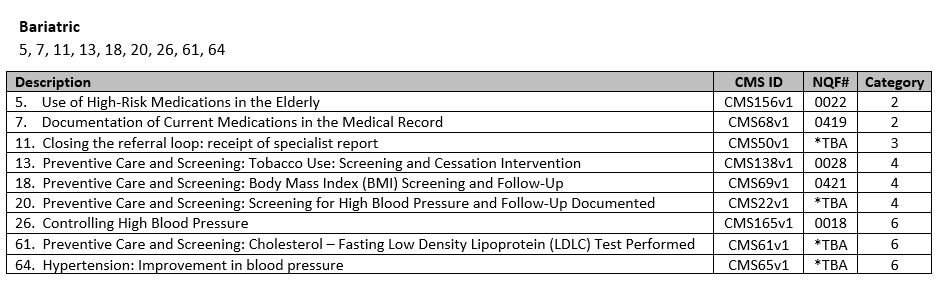
**Nextech is working with the American Academy of Dermatology to get certified for more of the dermatology specific measures such as Melanoma and Biopsy reporting. If you choose to report these measures in 2015, you will have to report via a qualified registry. There is more information in the links below about other reporting options.
PQRS Resources
HERE ARE SOME RELATED ARTICLES YOU MAY FIND INTERESTING
Regulatory & Compliance | Revenue & Finances
What Is PECOS? Step-by-Step Guidance for Specialty Practice Owners
By: Nextech | January 5th, 2026
MedSpa | Regulatory & Compliance | Aesthetics
IV Therapy Laws by State
By: Nextech | April 10th, 2025
MedSpa | Regulatory & Compliance | Aesthetics | podcast
The Current & Future State of the Medical Spa Business with Alex Thiersch
By: Tyler Terry | April 24th, 2024
